
Meet the X-shredder
Test-tube mosquitoes might help us beat malaria
Genetic editing might help us wipe out the disease.

It’s Mosquito Week again on the Gates Notes. This year I’m exploring some of the science behind malaria and other mosquito-borne diseases. You can read below about how gene editing could play a key role in eradicating malaria. I’ve also written about amazing advances in tracking the disease and how the parasite is a deadly shapeshifter.
Humans have spent thousands of years inventing new ways to kill mosquitoes. The Romans did it by draining swamps. Today you might have a bug zapper in your back yard. In low- and middle-income countries, it’s common to see people spraying insecticides or setting up sticky traps baited with sugar.
But evolution is smart. It is one-upping us by creating mosquitoes that are harder to kill. In sub-Saharan Africa and parts of South America and southeast Asia, we are seeing an alarming number of mosquitoes that can withstand insecticides.
This is especially problematic for the fight against mosquito-borne diseases like malaria. To eradicate these diseases, we need new tools to complement the ones we already have.
Our foundation is backing a lot of different advances. One that I’m especially excited about is a set of techniques for genetically modifying mosquitoes that could dramatically reduce the number of disease-carrying insects in certain areas.
What is cool about these genetic techniques is how precise they can be. Precision matters because out of more than 3,000 species of mosquitoes, only five are responsible for causing most cases of malaria. Of those, only females spread the disease, because they’re the only ones that bite humans. (They do it when they need extra protein for reproduction. Experts call it “taking a blood meal.”) The males just drink nectar.
The promise of gene editing is that, instead of killing a bunch of mosquitoes indiscriminately, we could eliminate only the dangerous ones in a particular area. That would buy us time to cure all the people there of malaria. Then we could let the mosquito population return without the parasite.
One exciting gene-editing technique is called gene drive. The term covers several different approaches, but the basic idea is to use the CRISPR method to rewrite the usual rules of inheritance. Normally, for any given gene, there’s a 50 percent chance that a parent with that gene will pass it on to a child. (It is competing with one from the other parent, and only one of the two can win.) With gene drive, the odds go up to 100 percent. You give a few mosquitoes an edited gene that inserts—or drives—itself into all their offspring. When those mosquitoes mate with wild mosquitoes, all their children will have the edited gene, and over time it will make its way through the entire population.
Imagine if blue-eyed mosquitoes had only blue-eyed children, no matter what color their partners’ eyes were. Eventually, every mosquito in that population would have blue eyes.
This chart shows you how gene drive eventually spreads a gene throughout an entire population:
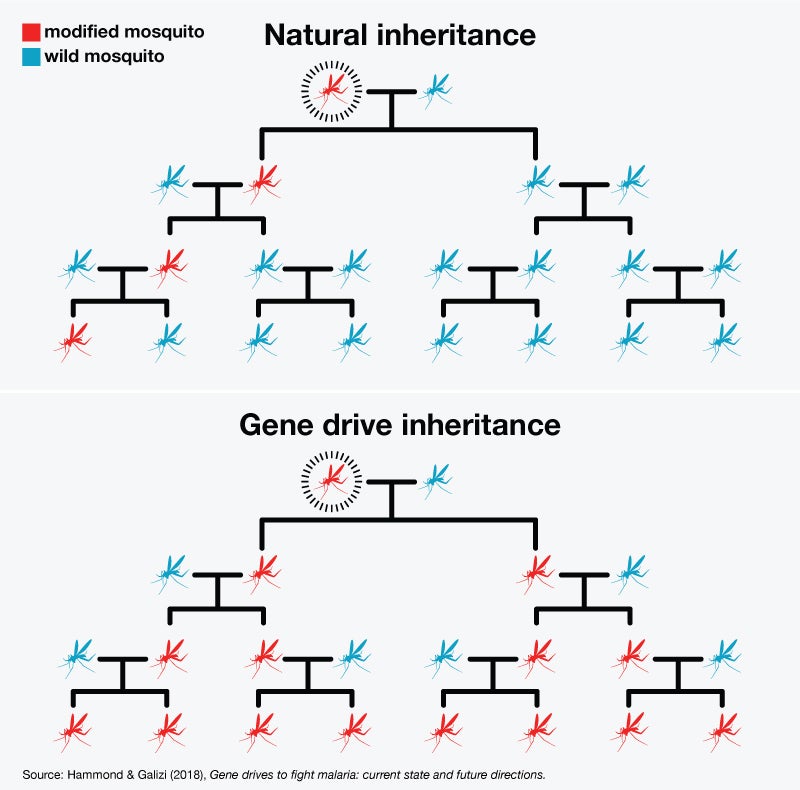
There’s no reason to think gene drive is even feasible in humans, let alone advisable. There are also serious questions surrounding the use of this technology on insects, which I will get to in a moment. But first I want to give you two examples of how it works.
One is the colorfully named X-shredder. As you might remember from biology class, the sex of a mosquito is determined partly by the sex chromosomes it inherits from its parents. Females got one X chromosome from each parent; males got an X from their mother and a Y from their father.
In 2014, scientists at Imperial College London and the Fred Hutchinson center here in Seattle were able to edit a protein in male mosquitoes so that it shreds the X chromosomes in their sperm. As a result, the males pass along mostly Y chromosomes, so most of their offspring will be males. Thanks to gene drive, those offspring will also have the edited protein, so most of their children will be males.
Within a few generations, the male/female ratio gets out of whack, and eventually the species dies off in that area.
Another example involves the doublesex gene, which in mosquitoes works along with the sex chromosome to determine whether an insect turns out male or female. Last year, researchers at Imperial College London found that females with edited doublesex genes develop a mix of male and female organs, including male genitalia and a proboscis that is too flimsy to break human skin. They can’t reproduce, so the population shrinks; and they can’t take a blood meal, so they won’t spread the parasite.
The doublesex edit doesn’t affect males, although thanks to gene drive, they will pass it to their offspring, which is how it keeps spreading through the population.
We know gene-drive technology works in the lab. When the Imperial College researchers put 150 males carrying a copy of the doublesex edit in a small cage with 450 wild-type mosquitoes, the population died off within a few months (about 10 generations). The sex bias edit produced similar results.
The next step is to run tests in larger cages and, eventually, get permission from governments to do them outdoors. We need to understand things like: What’s the impact on the food chain if a certain species of mosquito starts dying off? How many altered insects would we need to introduce? How long do we need the mosquitoes to be gone? Last year, the government of Burkina-Faso agreed to allow the release of sterile, non-gene-drive mosquitoes in the wild so researchers could begin to study some of these questions.
As I mentioned, social and regulatory issues also come into play. For example, because mosquitoes don’t exactly respect national boundaries, neighboring countries will probably need to agree on the rules surrounding the use of gene-editing technology. Policymakers and scientists have been debating these questions in forums like the World Health Organization and the African Union’s development agency, and they are moving toward a consensus.
I think we can have the regulatory approvals in place by 2024 and the first gene-drive mosquitoes ready for use by 2026. Although this technique will never replace the other tools we have for fighting malaria, I’m optimistic that it could become one more important weapon in eradicating the disease.


